Industry-Leading, Audit-Ready EDC
Expand your eClinical Ecosystem
Built to expand your eClinical suite — RealWorld EDC is an internationally trusted, vendor-ready EDC platform, with 15 years of proven success in clinical trials.
Schedule a Demo















































The Platform
Quality
Dual-layer Quality Controls
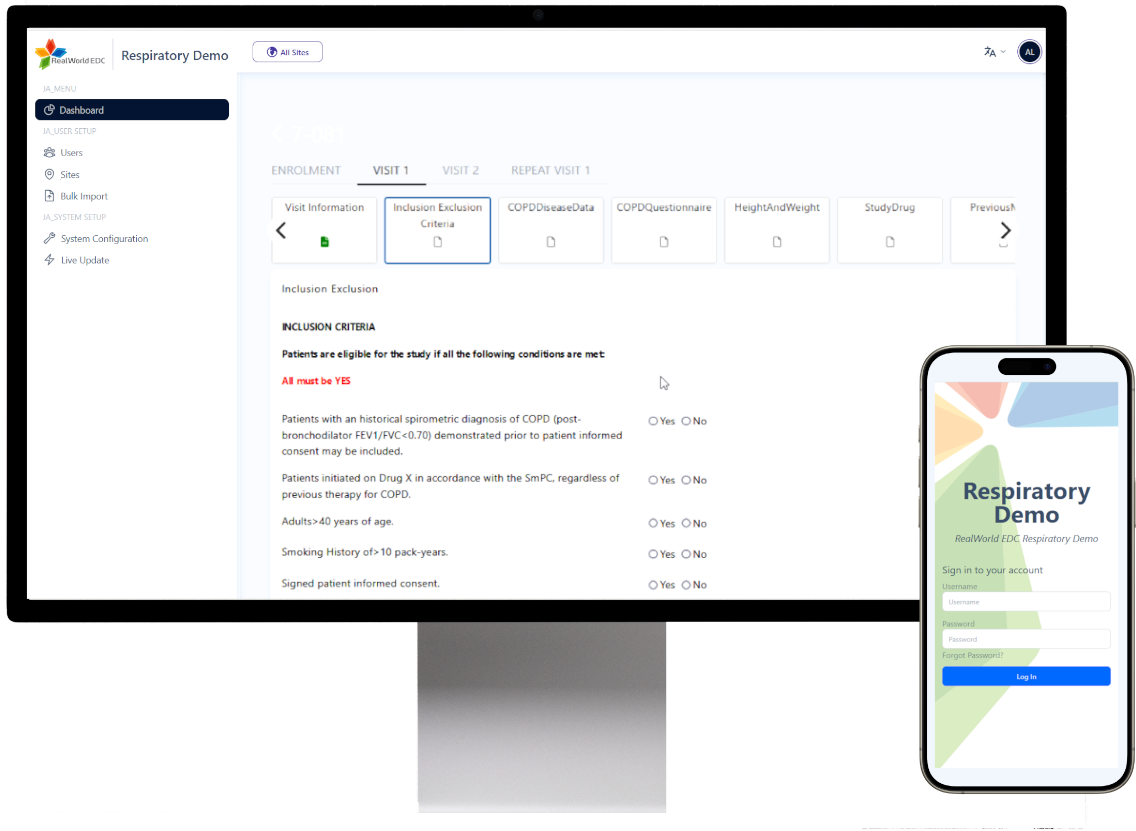
Instant edit checks built into every eCRF field. Configurable rule sets run in the background by data managers, combined with role-based workflows and full audit trails keep data clean and inspection-ready.
Security
Secure, Scalable Hosting
Deployed on ISO 27001-certified AWS infrastructure with encryption at rest and in transit, and an architecture engineered for 99.9% availability.
Confidence
Standards by Design
Natively stores study metadata and clinical data in CDISC ODM. One-click SAS and ODM XML exports keep statisticians and regulators in sync.
Fluid
Any Device, Any Language
Responsive UI and full Unicode support mean sites work on desktop, tablet or mobile in every major language.
Our Solutions
Efficient Study Build
Build or import study metadata using CDISC ODM.
Fast Onboarding and Training
Custom training modules facilitate fast site certification.
Clear Role-based Dashboards
Easily view the KPIs that matter.
Adaptive eCRF Design
Edit or extend forms post-go-live with zero downtime.
Advanced Data Management
Comprehensive edit-checks shorten query cycles.
Audit-ready Compliance
ODM certified. Proven compliance with industry standards and regulations.
Endorsed by Global Clinical Trial Leaders

“Very professional, helpful and responsive. Well done to you and RealWorld EDC on the very professional approach, getting a good application in on time, and particularly the follow‑up on the queries.”
Medical Director, Novartis

“There is no doubt that RealWorld EDC resources have been of great assistance to our local clinical‑trial programmes over the years.”
General Manager, Roche

“We had no problems with RealWorld EDC’s screen configuration and feel of use….the system was very good and met our expectations.”
Technology Leader, CMIC Japan

“RealWorld EDC is an outstanding solution.”
CEO, Seven to One CRO, Japan

“The RealWorld EDC system was quick — meets expectations for reliability, easy to use and value.”
Procurement Manager, Chugai Roche, Japan

“I think you designed a very clear and friendly eCRF and I’m happy to initiate sites with it.”
Project Manager, VLS Worldwide

“The support provided has been over and above what was expected. RealWorld EDC’s understanding of the NIS arena and their contact has been exceptional.”
Project Manager, Astellas

“I would rate the organisation and its people very highly, a dedicated team of professionals committed to excellent service levels.”
Area Commercial Director, Biogen

“We received final data within weeks of LP/LV and within two days of database lock.”
Executive, Precision Biotics

“RealWorld EDC was always willing to adapt the CRF and solve problems immediately. Our customers were especially satisfied with the quick and competent support.”
Executive, JSW Lifesciences (QPS)

“I was an EDC‑cynic until I used RealWorld EDC’s system and was delighted to have fewer CRA visits and no queries post LP/LV completion.”
Clinical Research Coordinator, Tallaght University Hospital

“RealWorld EDC not only made suggestions to help our Phase IV study, they delivered results fast.”
Medical Director, Servier

“RealWorld EDC has helped my research unit to become more efficient in meeting sponsor timelines; my staff loved it.”
Professor, Tallaght University Hospital
RealWorld EDC is Searching for Strategic Partners
RealWorld EDC invites organisations who require an EDC platform to augment their service offering and grow their clinical trial business.
RealWorld EDC is a novel EDC solution designed to streamline and optimise clinical trial management. With a track record of over 15 years and deployment in more than 80 clinical studies for leading global pharmaceutical companies, RealWorld EDC has consistently demonstrated its reliability, flexibility, and effectiveness.
Following a successful collaboration with Japan’s leading CRO, RealWorld EDC has been re-engineered, and the company is in search of clinical trial software vendors in the US and Europe whose business offering would benefit by the addition of RealWorld EDC.
A potential vendor would also have the opportunity to develop additional functionality, which would enhance their business offering.
A Platform that has Established a Global Reputation
Our multidisciplinary team lies at the core of our unique software solution. Over the past 15 years we have continually listened to our customers' needs which has driven the evolution of a leading EDC system.

Stephen Dorman
Chief Executive Officer

Tomas O'Mahoney
Director

Stephen Kennelly
Head Technology Strategist
Contact Us / Request Demo
Reach out for a free demo and we’ll get back to you as soon as possible.